September 14, 2022, Vientiane, Laos--BioSpring ("BioSpring Group.LTD" "BioSpring Group") launches New Product Packaging for SaiLin's Global Market Customers.
This product packaging upgrade is to fulfill the previous promise of BioSpring's acquisition of SaiLin, and will continue to provide follow-up support and services to Sailin's global customers. The batch will use Biospring's anti-counterfeiting verification system.
| No. 1 | Saibriga |
|---|---|
| Brand name | Saibriga |
| Compositon | Brigatinib |
| Strength | 90mg |
| Form | Tablet |
| Quantity Unit | 90mg*30T/bottle |
| Date of manufacture | September.2022 |
| Product Verification | www.biospring.ltd |
| Licensor | Sailin biopharmaceutical (Laos) co., LTD |
| Manufacturer | BioSpring.Ltd.,Laos PDR |


| No. 2 | Saibru |
|---|---|
| Brand name | Saibru |
| Compositon | Ibrutinib |
| Strength | 140mg |
| Form | Capsule |
| Quantity Unit | 140mg*90C/bottle |
| Date of manufacture | September.2022 |
| Product Verification | www.biospring.ltd |
| Licensor | Sailin biopharmaceutical (Laos) co., LTD |
| Manufacturer | BioSpring.Ltd.,Laos PDR |


| No. 3 | Saicabo |
|---|---|
| Brand name | Saicabo |
| Compositon | Cabozantinib |
| Strength | 20mg |
| Form | Capsule |
| Quantity Unit | 20mg*90C/bottle |
| Date of manufacture | September.2022 |
| Product Verification | www.biospring.ltd |
| Licensor | Sailin biopharmaceutical (Laos) co., LTD |
| Manufacturer | BioSpring.Ltd.,Laos PDR |


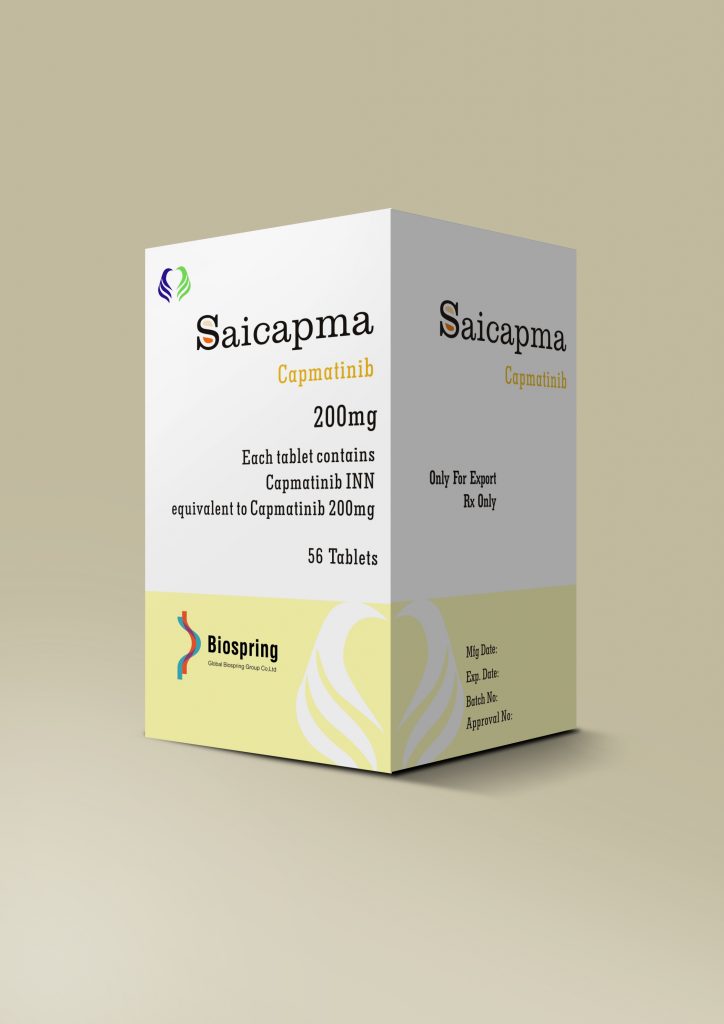
| No. 4 | Saicapma |
|---|---|
| Brand name | Saicapma |
| Compositon | Capmatinib |
| Strength | 200mg |
| Form | Tablet |
| Quantity Unit | 200mg*56T/bottle |
| Date of manufacture | September.2022 |
| Product Verification | www.biospring.ltd |
| Licensor | Sailin biopharmaceutical (Laos) co., LTD |
| Manufacturer | BioSpring.Ltd.,Laos PDR |

| No. 5 | Saicrizo |
|---|---|
| Brand name | Saicrizo |
| Compositon | Crizotinib |
| Strength | 250mg |
| Form | Capsule |
| Quantity Unit | 250mg*60C/bottle |
| Date of manufacture | September.2022 |
| Product Verification | www.biospring.ltd |
| Licensor | Sailin biopharmaceutical (Laos) co., LTD |
| Manufacturer | BioSpring.Ltd.,Laos PDR |


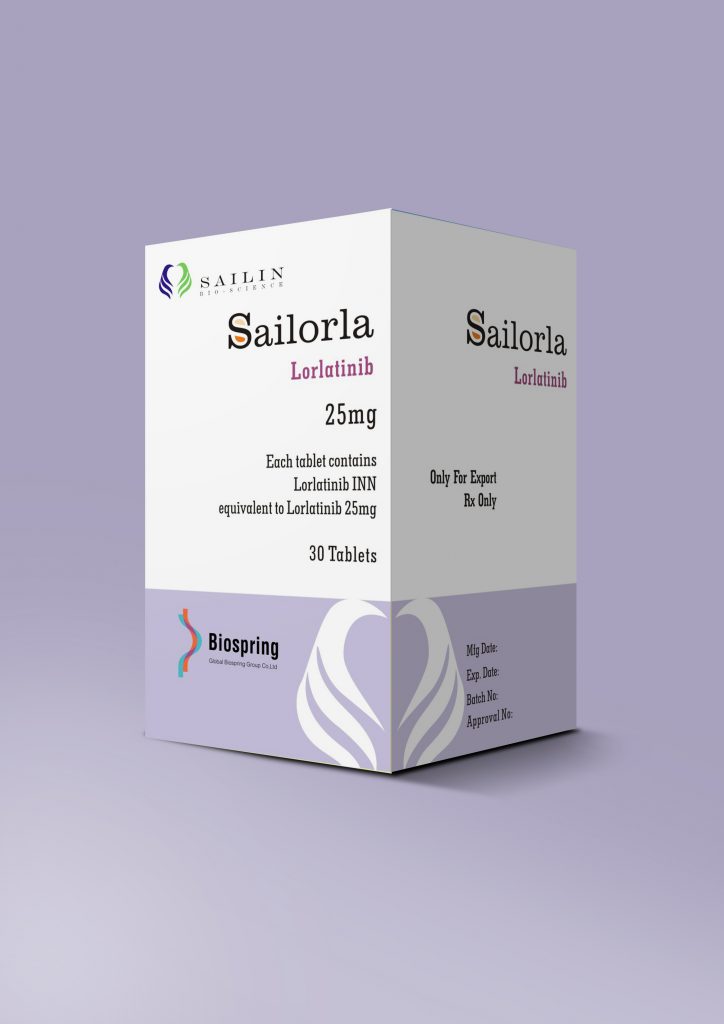
| No. 6 | Sailorla |
|---|---|
| Brand name | Sailorla |
| Compositon | Lorlatinib |
| Strength | 25mg |
| Form | Tablet |
| Quantity Unit | 25mg*30T/bottle |
| Date of manufacture | September.2022 |
| Product Verification | www.biospring.ltd |
| Licensor | Sailin biopharmaceutical (Laos) co., LTD |
| Manufacturer | BioSpring.Ltd.,Laos PDR |
| No. 7 | Saiosimer |
|---|---|
| Brand name | Saiosimer |
| Compositon | Osimertinib |
| Strength | 80mg |
| Form | Tablet |
| Quantity Unit | 80mg*30T/bottle |
| Date of manufacture | September.2022 |
| Product Verification | www.biospring.ltd |
| Licensor | Sailin biopharmaceutical (Laos) co., LTD |
| Manufacturer | BioSpring.Ltd.,Laos PDR |


About BioSpring.Ltd
Founded in 2022 at the Vientiane Saiseta Comprehensive Development Zone in Laos, BioSpring is the country's fastest growing generic drug manufacturing company and the country's first pharmaceutical company targeting the international market, serving Asia, Africa, Europe and Latin America Americas and many other countries and regions.
About Sailin Biopharma
Sailin biopharmaceutical (Laos) co., LTD., and the construction in 2019, is currently the largest and the most technologically advanced Laos anticancer and antiviral drugs production enterprise joint pharmaceutical group co., LTD., a subsidiary of the country's fastest growing large pharmaceutical companies.
Sailin pharmaceutical is a pharmaceutical enterprise management practices, in vientiane Rosetta park covers an area of 60 mu, raw material production and preparation production is equal to one, the company has more than 152 employees.Visit: Lao pharmaceutical company BioSpring announces acquisition agreement with cancer drug company Sailin Biopharma - BioSpring.Ltd
About Pharmaceutical Third Party Manufacturing
Pharma Third party manufacturing/contract manufacturing provide easy solution for manufacturing of own brands.
Third party or contract manufacturing is referred to out sourcing of products or to get manufactured one’s brand names from others manufacturing unit out. In current time it is very popular concept among marketing companies. Even companies having their own manufacturing units are gotten manufactured their products from outside. Multinational companies are also gotten manufactured their products at loan license or third party basis.
Third Party/Contract Party Manufacturers Agency
RxLibra pharmaceutical Sole Co.,Ltd
Forward-Looking Statements
To the extent applicable laws and regulations, expressions such as "plan," "target," "expect," "forecast," and similar expressions appearing in this press release are forward-looking statements. The forward-looking statements set forth herein involve a number of risks and uncertainties that may differ materially from actual results. Important factors leading to such differences include, but are not limited to, changes in regulations and/or economic conditions, uncertainty in clinical research results, exposure to various market risks, and other factors beyond the control of the company.